Cancer Epidemiology Biomarkers & Prevention
Vol. 10, 1155-1163, November 2001
© 2001
American Association for Cancer Research
Non-Hodgkin’s Lymphoma and Specific Pesticide Exposures in Men
Cross-Canada Study of Pesticides and Health1
Helen H. McDuffie2,
Punam Pahwa, John R. McLaughlin,
John J. Spinelli, Shirley Fincham,
James A. Dosman, Diane Robson,
Leo F. Skinnider and Norman W. Choi3
Centre for Agricultural Medicine,
University of Saskatchewan, Saskatoon, Saskatchewan, S7N 0W8
[H. H. M., P. P., J. A. D.]; National Cancer Institute of
Canada, Epidemiology Unit, University of Toronto, Toronto,
Ontario, M5S 1A8 [J. R. M.]; Centre for Health Evaluation and
Outcome Sciences, St. Pauls Hospital, Vancouver, British
Columbia, V6Z 1Y6 [J. S.]; Alberta Cancer Board, Division of
Epidemiology, Prevention and Screening, Edmonton, Alberta, T6G
1Z2 [S. F.]; Saskatchewan Cancer Agency, Allan Blair Memorial
Centre, Regina, Saskatchewan, S4T 7T1 [D. R.]; Department of
Pathology, University of Saskatchewan, Saskatoon,
Saskatchewan, S7N 0W8 [L. F. S.]; and Manitoba Cancer
Treatment and Research Foundation, Winnipeg, Manitoba, R3E 0V9
[N. W. C.], Canada
 |
Abstract |
Our objective in the study was to investigate the putative
associations of specific pesticides with
non-Hodgkin’s Lymphoma [NHL; International
Classification of Diseases, version 9 (ICD-9) 200,
202]. We conducted a Canadian multicenter population-based
incident, case (n = 517)-control (n =
1506) study among men in a diversity of occupations
using an initial postal questionnaire followed by a
telephone interview for those reporting pesticide
exposure of 10 h/year or more, and a 15% random sample of the
remainder. Adjusted odds ratios (ORs) were computed
using conditional logistic regression stratified by
the matching variables of age and province of
residence, and subsequently adjusted for
statistically significant medical variables (history of
measles, mumps, cancer, allergy desensitization
treatment, and a positive history of cancer in
first-degree relatives). We found that among major
chemical classes of herbicides, the risk of NHL was
statistically significantly increased by exposure to
phenoxyherbicides [OR, 1.38; 95% confidence
interval (CI), 1.06–1.81] and to dicamba (OR, 1.88;
95% CI, 1.32–2.68). Exposure to carbamate (OR,
1.92; 95% CI, 1.22–3.04) and to organophosphorus
insecticides (OR, 1.73; 95% CI, 1.27–2.36), amide fungicides,
and the fumigant carbon tetrachloride (OR, 2.42; 95% CI,
1.19–5.14) statistically significantly increased
risk. Among individual compounds, in multivariate
analyses, the risk of NHL was statistically
significantly increased by exposure to the herbicides
2,4-dichlorophenoxyacetic acid (2,4-D; OR, 1.32;
95% CI, 1.01–1.73), mecoprop (OR, 2.33; 95% CI,
1.58–3.44), and dicamba (OR, 1.68; 95% CI,
1.00–2.81); to the insecticides malathion (OR, 1.83; 95%
CI, 1.31–2.55), 1,1,1-trichloro-2,2-bis (4-chlorophenyl)
ethane (DDT), carbaryl (OR, 2.11; 95% CI, 1.21–3.69),
aldrin, and lindane; and to the fungicides captan and
sulfur compounds. In additional multivariate
models, which included exposure to other major
chemical classes or individual pesticides, personal
antecedent cancer, a history of cancer among first-degree
relatives, and exposure to mixtures containing dicamba
(OR, 1.96; 95% CI, 1.40–2.75) or to mecoprop (OR,
2.22; 95% CI, 1.49–3.29) and to aldrin (OR, 3.42;
95% CI, 1.18–9.95) were significant independent
predictors of an increased risk for NHL, whereas a
personal history of measles and of allergy
desensitization treatments lowered the risk. We concluded that
NHL was associated with specific pesticides after
adjustment for other independent predictors.
 |
Introduction |
NHL4 has been epidemiologically
associated with farming (1,
2, 3, 4,
5, 6, 7,
8) , with certain farm practices
(9) , with pesticide exposure
(10, 11,
12, 13) , and with certain other
occupations (14, 15,
16, 17) . The term
pesticide is used to denote a wide variety of
chemicals used to destroy weeds (herbicides),
insects (insecticides), and mold (fungicides). Such chemicals
are widely used in agriculture, horticulture, and
forestry, and in the secondary processing of the
products of these primary industries. Many of the
NHL and pesticide case-control or cohort studies
focused either on a small geographical area (1
, 2 , 4) or on
one occupational group (2 , 4
, 5 , 9) . Our study
encompassed six provinces of Canada with diverse
agricultural practices and a number of different
types of occupational and nonoccupational exposures
to pesticides. Non-Hodgkin’s lymphoma incidence
rates have been increasing in Canada for the last
25 years reflecting a worldwide trend (18)
that has not been explained by improved diagnostic
(19) methods or record-keeping
(20) .
 |
Materials and Methods |
Study Population.
We conducted a population-based case-control study among men
resident in six Canadian provinces to test the
pesticide-exposure hypothesis related to four rare
tumors. Incident cases among men, ages 19 years or
over, with a first diagnosis of STS, HD, NHL
[International Classification of Diseases, version 9 (ICD-9),
code 200 or 202], or MM diagnosed between September 1,
1991, and December 31, 1994, were eligible. To
balance the number of cases by geographical
regions, each province was assigned a target number
of cases in each tumor category. Each province
ceased to ascertain cases when their preassigned target was
reached. This report is based solely on cases diagnosed
with NHL. Cases were ascertained from provincial
Cancer Registries except in Quebec, for which
hospital ascertainment was used. The Cancer
Registries and hospitals provided information, including
pathology reports, to confirm the diagnosis.
Pathological material was reviewed and classified
according to the working formulation by the
reference pathologist. Misclassified and ineligible (e.g.,
Kaposi’s sarcoma, known HIV-positive) cases were
excluded. Subjects for whom pathological material
was unavailable remained in the study. After
physician consent was received, postal questionnaires
and informed consent forms were mailed to potential
cases. Surrogates for deceased cases were not
contacted.
Men, ages 19 years and older, selected at random within age
constraints from the provincial Health Insurance records
(Alberta, Saskatchewan, Manitoba, Quebec),
computerized telephone listings (Ontario), or
voters’ lists (British Columbia) were potential
controls. The random control subject selection was stratified
by age ± 2 years to be comparable with the age
distribution of the entire case group (STS, HD,
NHL, and MM) within each province. Postal
questionnaires and informed consent forms were
mailed to potential controls. Surrogates for deceased persons
were ineligible as controls. All of the participating
control subjects were used in the statistical
analyses of each cancer site.
Pilot Study.
We conducted a pilot study (21) in each
provincial region to test study procedures and to
determine an operational definition of pesticide
exposure to distinguish between environmental (which
includes bystander and incidental) and more intensive
exposure. Nonoccupational use of pesticides (home,
garden, hobby) was included. There were few
individuals who were completely free of being
exposed to pesticides. Therefore, we constructed graphs
that demonstrated that the most efficient definition of
pesticide exposure, which discriminated (a)
between incidental, bystander, and environmental
exposure as compared with more intensive exposure
and (b) between cases and controls, was a cumulative
total of 10 h per year to any combination of
pesticides. The screening questions in the postal
questionnaire were used to trigger telephone
interviews among those with cumulative exposure of
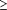 10 h/year
to any combination of herbicides, insecticides,
fungicides, fumigants, and/or algicides. The 68
cases and 103 controls who participated in the
pilot study are not included in this report. 10 h/year
to any combination of herbicides, insecticides,
fungicides, fumigants, and/or algicides. The 68
cases and 103 controls who participated in the
pilot study are not included in this report.
Pesticides.
Pesticide is a generic term describing a variety of compounds
of diverse chemical structures and biological modes of
action. In this study, the term pesticide refers
primarily to herbicides, insecticides, fungicides,
and fumigants.
We conducted a validation pilot study of the modified
questionnaires (21) . Volunteer
farmers (n = 27) completed the questionnaires
and granted permission for us to access their records of
purchases through their local agrochemical
supplier. The concordance between the two sources
was excellent and discordance was explainable by (a)
the farmer paid in cash and the supplier discarded the
record; (b) the farmer purchased the agrochemical
in the United States, and, therefore, the local
supplier did not have a record; (c) the
farmer paid for professional ground or aerial spraying,
and the account was listed in another name; or (d)
the supplier had destroyed the records.
Questionnaires.
The questionnaires were modified versions of the telephone
interview questionnaire that was used in studies of
pesticide exposure and rare tumors in Kansas
(11) and Nebraska (13) .
With permission, we modified the questionnaire to
create postal and telephone interview
questionnaires. To control for the effects of other
variables known or suspected to be associated with the
development of NHL after conducting an extensive
literature review, we used the postal questionnaire
to capture demographic characteristics, antecedent
medical history, family history of cancer, detailed
lifetime job history, and occupational exposure history to
selected substances, accidental pesticide spills,
and use of protective equipment, as well as details
of cigarette smoking history. The telephone
questionnaire characterized exposure to individual
pesticides. The pesticide data were collected at several
levels beginning with the broadest categories (e.g.,
minimal exposure, occupations with potential
pesticide exposure) and progressing sequentially to
major classes (e.g., herbicides); to chemical
groups (e.g., phenoxy herbicides); and finally to
individual compounds (e.g., 2,4-D, MCPA, and
2,4,5-T).
In this report, we focus on lifetime exposure to individual
pesticides classified by active ingredients and to major
chemical classes of herbicides, insecticides,
fungicides, and fumigants. We classified exposure
by the number of herbicides, insecticides,
fungicides, and fumigants reported by cases and controls as
well as by the number of days per year of exposure to
individual compounds.
Each subject who reported 10 h per year or more of exposure
to pesticides (any combination of compounds) as defined
by the screening questions, and a 15% random sample
of the remainder was mailed a list of pesticides
(both chemical and brand names) and an information
letter. Each subject was subsequently telephoned to
obtain details of pesticide use.
The listed pesticides were chosen for inclusion
(22, 23,
24, 25) : (a) if the
compound was ever registered for use in Canada and
reviewed by the IARC; (b) if the pesticide was recently
banned or restricted in Canada by the federal licensing
agency; or (c) if the pesticide was commonly
used in Canada for specific purposes.
To ensure consistency, we developed and distributed manuals
for provincial study coordinators, interviewers, and
data managers. Before commencing data collection,
we held a 2-day workshop with provincial
coordinators to review data collection procedures
and policies, to practice interviewing skills, and to review
SPSS-DE (Statistical Packages for the Social
Sciences-Data Entry),5 the
custom data entry program that we used. On receipt of a
postal questionnaire, the provincial coordinator
reviewed it for internal consistency and
completeness. Data were computer-entered and
verified in the province of origin, transported to the
coordinating center, and rechecked for
completeness, after which statistical analyses were
performed.
Copies of the questionnaires and additional information on
pesticides that were not included in this report
are available from the corresponding author.
Pathology Review.
Pathologists in participating provinces were requested to send
blocks or slides of tumor tissue removed at surgery to
the reference pathologist. Ten subjects with
Kaposi’s sarcoma were omitted on the basis of the
etiological association with HIV infection. Any
other known HIV-positive subjects had been previously
excluded. Eighty-four % (436 of 517) of the NHL
tumors were validated. Because of a change midstudy
in some hospitals’ policies regarding supplying
pathological material without charge, we were
unable to obtain the remaining samples.
Statistical Analyses.
Data from the postal and telephone interviews were merged by
using the identification number. Of the individuals
selected randomly for a telephone interview, most
had used one or no chemical pesticides. We reviewed
these data and decided to include them in the
statistical analyses because they might be informative
with respect to low levels of exposure to pesticides and
their inclusion maximized our sample size with
respect to other known or suspected risk factors
for NHL. We conducted descriptive analyses of each
variable, which included, where applicable,
frequencies, ranges, means ± SD, and median values for
cases and controls separately.
To evaluate putative risk factors for NHL, conditional
logistic regression was used to compute ORs and 95%
CIs, stratifying by age groups and province of
residence.6 ORs were calculated
for categorical variables related to medical history
that were selected based on previous studies (e.g.,
measles, mumps, previous cancer, allergy
desensitization treatment, skin prick allergy
test); pesticide exposure (<10 and
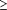 10 h per
year); and smoking history. Using conditional
logistic regression, ORs were also calculated for (a)
major chemical classes of herbicides, insecticides,
fungicides, and fumigants; and (b) for individual
active chemicals. The statistically significant (P
< 0.05) medical variables were used to adjust the
effect of exposure to pesticides classified by
major chemical group and by individual active chemical. Given
the study sample size and the case-control ratio, a
priori power calculations indicated that we had
sufficient statistical power to detect an OR of 2
when at least 1% of the controls was exposed to a
specific pesticide or chemical class of pesticide. Conditional
logistic analyses (26) were conducted
that retained in the model, all covariates for
which the P was 10 h per
year); and smoking history. Using conditional
logistic regression, ORs were also calculated for (a)
major chemical classes of herbicides, insecticides,
fungicides, and fumigants; and (b) for individual
active chemicals. The statistically significant (P
< 0.05) medical variables were used to adjust the
effect of exposure to pesticides classified by
major chemical group and by individual active chemical. Given
the study sample size and the case-control ratio, a
priori power calculations indicated that we had
sufficient statistical power to detect an OR of 2
when at least 1% of the controls was exposed to a
specific pesticide or chemical class of pesticide. Conditional
logistic analyses (26) were conducted
that retained in the model, all covariates for
which the P was
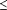 .05. The
criterion for entry into models was a P .05. The
criterion for entry into models was a P
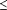 0.20 in
bivariate age and province stratified analyses. 0.20 in
bivariate age and province stratified analyses.
We created dose-response levels based on days/year of
personally mixing or applying selected herbicides,
insecticides, fungicides, and fumigants. We
reported ORs stratified by age and province of
residence. We created exposure categories for exposures to
multiple different herbicides, insecticides, fungicides,
and fumigants. For these analyses, the unexposed
category was specific to the class of pesticide. We
also created exposure categories for exposures to
combinations of herbicides, insecticides, fungicides,
and fumigants for which the reference group did not
report exposure to any of those classes of
pesticides.
Ethics.
The protocol, letters of informed consent, questionnaires, and
all other correspondence with potential subjects were
approved by the relevant agencies in each province.
All of the information that could be used to
identify individuals remained within the province
of origin under the control of the provincial principal
investigators.
 |
Results |
Data from postal questionnaires based on responses from 517
NHL cases (67.1% of those contacted) and 1506 control
subjects (48.0% of those contacted) were analyzed.
Similar percentages of potential subjects resident
in rural and urban areas responded. There were
higher percentages of responders in the middle-age
group than at either extreme among both cases and controls.
Detailed information related to their pesticide exposure
history was obtained by telephone interview from
119 NHL cases and 301 control subjects who
indicated pesticide exposure of 10 h per year or
more. A 15% random sample of cases and controls who
indicated pesticide exposure of less than 10 h/year was also
interviewed by telephone, resulting in detailed
pesticide exposure information on 60 cases of NHL
and on 155 controls. The total telephone
interviewed sample consisted of 179 cases of NHL and
456 controls.
A summary of selected demographic, antecedent personal and
familial medical history, general pesticide
exposure as measured by the screening questions,
and cigarette smoking history comparisons of NHL
cases and population-based controls is shown in Table
1 . Because all of the controls (age-matched for STS, MM, HD,
and NHL) were used in the analysis, cases were older
than controls. Cases and controls were similar in
their smoking patterns. Cases were less likely to
have a history of measles or mumps and more likely
to have a personal history of a previous primary cancer.
Cases were more likely than controls to have a positive
family history of cancer, whereas more controls had
undergone allergy desensitization injections. A
slightly higher proportion of cases than controls
indicated cumulative exposure to pesticides of
. Because all of the controls (age-matched for STS, MM, HD,
and NHL) were used in the analysis, cases were older
than controls. Cases and controls were similar in
their smoking patterns. Cases were less likely to
have a history of measles or mumps and more likely
to have a personal history of a previous primary cancer.
Cases were more likely than controls to have a positive
family history of cancer, whereas more controls had
undergone allergy desensitization injections. A
slightly higher proportion of cases than controls
indicated cumulative exposure to pesticides of
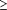 10 h per
year. 10 h per
year.
View this table:
[in this window]
[in a new window]
|
Table 1 Comparisons of
demographic, antecedent personal medical, general
pesticide exposures and cigarette smoking history
between cases of NHL and control subjects based on the
postal questionnaire
|
|
Table 2 summarizes reported exposure to herbicides classified
by major chemical classes (phenoxy, phosphonic acid,
thiocarbamates, phenols, dicamba, and
dinitroaniline) and by individual compounds for
which at least 1% of responders reported exposure. ORs are
also shown after adjustment for the statistically
significant (P < 0.05) variables reviewed in
Table 1
summarizes reported exposure to herbicides classified
by major chemical classes (phenoxy, phosphonic acid,
thiocarbamates, phenols, dicamba, and
dinitroaniline) and by individual compounds for
which at least 1% of responders reported exposure. ORs are
also shown after adjustment for the statistically
significant (P < 0.05) variables reviewed in
Table 1 , which included a history of measles, mumps,
cancer, and allergy desensitization shots and a
positive history of cancer in a first-degree relative.
Cases experienced a significantly higher frequency of
exposure to phenoxyherbicides, to dicamba or a
mixture including dicamba, to 2,4-D, and to
mecoprop.
, which included a history of measles, mumps,
cancer, and allergy desensitization shots and a
positive history of cancer in a first-degree relative.
Cases experienced a significantly higher frequency of
exposure to phenoxyherbicides, to dicamba or a
mixture including dicamba, to 2,4-D, and to
mecoprop.
View this table:
[in this window]
[in a new window]
|
Table 2 Herbicides:
frequency of exposure to herbicides classified into
major chemical classes and as individual compounds
|
|
Table 3 summarizes the insecticide exposure data. Exposure
to two major chemical classes, carbamates and
organophosphates, was statistically significantly
associated with NHL, whereas exposure to
organochlorines as a group was not. Among individual
carbamate compounds, exposure to carbaryl was
statistically significantly associated with NHL.
Among organochlorines, exposure to lindane, to
aldrin, and to DDT was significantly associated
with NHL. Malathion was the only individual organophosphate
exposure statistically significantly associated with
NHL.
summarizes the insecticide exposure data. Exposure
to two major chemical classes, carbamates and
organophosphates, was statistically significantly
associated with NHL, whereas exposure to
organochlorines as a group was not. Among individual
carbamate compounds, exposure to carbaryl was
statistically significantly associated with NHL.
Among organochlorines, exposure to lindane, to
aldrin, and to DDT was significantly associated
with NHL. Malathion was the only individual organophosphate
exposure statistically significantly associated with
NHL.
View this table:
[in this window]
[in a new window]
|
Table 3 Insecticides:
frequency of exposure to insecticides classified into
major chemical classes and as individual compounds
|
|
Exposure to fungicides is summarized in Table 4 . The fungicides with an amide group (ORadj,
1.70; 95% CI, 1.04–2.78) were associated with NHL,
whereas aldehydes and those containing mercury were
not. Among individual amidecontaining compounds,
exposure to captan (ORadj, 2.51; 95% CI, 1.32–4.76)
was associated with NHL.
. The fungicides with an amide group (ORadj,
1.70; 95% CI, 1.04–2.78) were associated with NHL,
whereas aldehydes and those containing mercury were
not. Among individual amidecontaining compounds,
exposure to captan (ORadj, 2.51; 95% CI, 1.32–4.76)
was associated with NHL.
View this table:
[in this window]
[in a new window]
|
Table 4 Fungicides:
frequency of exposure to fungicides classified into
major chemical classes and as individual compounds
|
|
Malathion used as a fumigant was not associated with NHL
(Table 5) . There were fewer users of malathion as a fumigant compared
with its use on crops. Carbon tetrachloride fumigant
exposure (ORadj, 2.42; 95% CI,
1.19–5.14) was associated with NHL.
. There were fewer users of malathion as a fumigant compared
with its use on crops. Carbon tetrachloride fumigant
exposure (ORadj, 2.42; 95% CI,
1.19–5.14) was associated with NHL.
Table 6 shows the results of a conditional logistic regression
model that included major chemical classes of pesticides
and all other covariates for which P < 0.05.
The variables that remained statistically
significantly associated with increased risk of NHL
were a previous personal history of another malignancy,
a history of cancer among first-degree relatives, and
exposure to dicamba and mixtures containing
dicamba. ORs for a personal history of measles or
of allergy desensitization injections were
significantly lower than those without this history. Table
7
shows the results of a conditional logistic regression
model that included major chemical classes of pesticides
and all other covariates for which P < 0.05.
The variables that remained statistically
significantly associated with increased risk of NHL
were a previous personal history of another malignancy,
a history of cancer among first-degree relatives, and
exposure to dicamba and mixtures containing
dicamba. ORs for a personal history of measles or
of allergy desensitization injections were
significantly lower than those without this history. Table
7 summarizes a similar model that included individual pesticides
and all of the other covariates for which P <
0.05 and in which mecoprop and aldrin exposure as
well as the same covariates as in Table 6
summarizes a similar model that included individual pesticides
and all of the other covariates for which P <
0.05 and in which mecoprop and aldrin exposure as
well as the same covariates as in Table 6 were associated with NHL.
were associated with NHL.
View this table:
[in this window]
[in a new window]
|
Table 6 Most
parsimonious model: conditional logistic regression
analyses that contained major chemical classes of
pesticides and important covariates (P < 0.05)
|
|
View this table:
[in this window]
[in a new window]
|
Table 7 Most
parsimonious model: conditional logistic regression
analyses that contained individual chemical pesticides
and important covariates (P < 0.05)
|
|
Table 8 shows the frequency of exposure to selected individual
herbicides, insecticides, fungicides, and fumigants,
stratified by the average number of days per year
of exposure. In general, the results of these
dose-response analyses are consistent with the
exposed/nonexposed findings. Those compounds for which we
found statistically significant case-control differences
also have elevated ORs based on strata of the
variable "days per year of exposure" (mecoprop,
dicamba, malathion, DDT, captan, carbon
tetrachloride, and sulfur). The exceptions were 2,4-D,
for which there was no dose-response relationship, and
glyphosate, which was not significant for exposure
but for which we demonstrated a dose-response
relationship.
shows the frequency of exposure to selected individual
herbicides, insecticides, fungicides, and fumigants,
stratified by the average number of days per year
of exposure. In general, the results of these
dose-response analyses are consistent with the
exposed/nonexposed findings. Those compounds for which we
found statistically significant case-control differences
also have elevated ORs based on strata of the
variable "days per year of exposure" (mecoprop,
dicamba, malathion, DDT, captan, carbon
tetrachloride, and sulfur). The exceptions were 2,4-D,
for which there was no dose-response relationship, and
glyphosate, which was not significant for exposure
but for which we demonstrated a dose-response
relationship.
View this table:
[in this window]
[in a new window]
|
Table 8 Frequency of
exposure to selected herbicides, insecticides,
fungicides, and fumigants stratified by the number of
days per year of exposure
|
|
Table 9 compares the frequencies of multiple herbicide, insecticide,
fungicide, and fumigant use among cases and controls.
Cases are significantly more likely to report
exposure to between two and four herbicides or
insecticides but not to five and more of either. An
elevated OR was found for exposure to two or more
fungicides. Table 9
compares the frequencies of multiple herbicide, insecticide,
fungicide, and fumigant use among cases and controls.
Cases are significantly more likely to report
exposure to between two and four herbicides or
insecticides but not to five and more of either. An
elevated OR was found for exposure to two or more
fungicides. Table 9 also shows a dose-response relationship in
comparisons of subjects who reported no pesticide exposure
and those who reported using five or more pesticides.
also shows a dose-response relationship in
comparisons of subjects who reported no pesticide exposure
and those who reported using five or more pesticides.
 |
Discussion |
The hypothesis that farming (1,
2, 3, 4,
5, 6, 7,
8) , agricultural practices
(9) , and pesticide exposure
(10, 11, 12,
13 , 22,
23, 24,
25) are associated with NHL has been tested in a number
of occupational studies. Not all of the studies confirm
an association (27,
28, 29) . Pesticides
have diverse chemistry and biological modes of
action. In addition to the active ingredients, there
are emulsifiers, carriers, dispersants, and a variety of
agents used to formulate liquids, granular and
mists. The major chemical classes of a priori
interest based on epidemiological studies
(10, 11,
12, 13 , 22,
23, 24,
25) were phenoxyherbicides, organophosphorus,
organochlorines, aldehydes, and carbon tetrachloride.
Occupational exposure to 2,4-D, 2,4,5-T, carbaryl,
chlordane, DDT, diazinon, dichlorvos, lindane,
malathion, nicotine, and toxaphene has been
reported to be associated with NHL. In addition, our interest
focused on pesticides classified as possibly or probably
carcinogenic to humans based on evaluations by the
IARC expert panels (Refs. 22,
23, 24,
25 ; phenoxyherbicides including 2,4-D, MCPA, and
2,4,5-T as a group, atrazine, chlordane, DDT,
dichlorvos, heptachlor, and pentachlorophenol). Our
bivariate results for exposure to groups of
phenoxyherbicides or dicamba-containing herbicides,
for carbamates and organophosphorus insecticides, and for
amide fungicides and for carbon tetrachloride were
not attenuated when simultaneously adjusted for the
important medical covariates (history of measles,
mumps, cancer, allergy desensitization shots, and a
positive history of cancer in a first-degree relative).
Among individual compounds, our results that related to
exposure to 2,4-D, mecoprop, dicamba, malathion,
DDT, carbaryl, lindane, aldrin, captan, and sulfur
compounds were not attenuated after simultaneous
adjustment for the same medical covariates. Clearly,
we had few exposed men whose exposure was limited to one
pesticide or to one class of pesticides. Our
results show elevated risk for exposure to multiple
herbicides, insecticides, and fungicides.
The strength of our results is enhanced by their internal
consistency as we applied the strategy of assessing
risk by different analytic approaches progressing
from exposure to: (a) major chemical classes
of herbicides, insecticides, fungicides, and fumigants;
(b) individual compounds within those major
chemical classes; and (c) individual
compounds stratified by days per year of exposure.
We constructed models that included potential confounders
(e.g., positive history of cancer in a
first-degree relative). Generally, the same
individual compounds or class of compounds was
associated with case status. The risk estimates based on
exposure to major chemical classes or to individual
compounds tended to be precise, as indicated by the
95% CIs.
Our results confirm previously reported associations of NHL
and a personal history of cancer (30
, 31) , of NHL and a history of
cancer among first-degree relatives (32
, 33) , and of NHL and exposure
to selected pesticides (1 , 3
, 5 , 9, 10,
11, 12,
13) . We were unable to find a previous
report suggesting a protective effect of allergy
desensitization shots. Koepsell et al.
reported little association of the number of allergy
desensitization shots and MM (34) .
The relationship between allergy and cancer is
complex with well-designed studies reporting
opposite results (35, 36,
37, 38) . Cigarette
smoking was not a risk factor overall, confirming
one study (39) and contradicting
others (40 , 41) ,
although certain subtypes (39
, 40) of NHL may be associated
with cigarette smoking.
The limitations of this study relate to those inherent in
the case-control design, specifically the potential
for recall bias and for misclassification of
pesticide exposure. Hoar et al. and Zahm
et al. (11 , 13) ,
as well as others (27, 28,
29 , 42,
43, 44,
45) , have dealt extensively with these issues among
farmers. We have included individuals in many different
occupations as well as home and garden users. These
are groups for whom we did not find extensive
validation studies. Their inclusion may have biased
our dose-response findings toward the null,
although the yes/no responses to individual pesticides would
be less affected. We reduced the number of surrogate
responders by excluding deceased persons from our
definition of eligible subjects. This strategy was
useful in decreasing the potential for
misclassification of exposure.
A second limitation is the less-than-optimal response
rates. We continued to recruit subjects in each
province until the target numbers were achieved. We
compared respondents to nonrespondents using postal
codes as an indicator of rural residence, and we
did not find a rural bias among respondents.
We reported results for a number of chemical agents and
exposures, not all of which were specified in the
hypothesis. Therefore, the statistical analyses
related to these unspecified agents should be
considered exploratory. As a consequence of conducting
multiple comparisons, a small number of statistically
significant results may be attributable to chance.
The two-tiered study design permitted us to obtain detailed
information related to factors other than pesticides
that are known or suspected of being etiologically
associated with NHL. The mailing of a list of
pesticides with both trade and generic chemical
names followed by a telephone interview allowed the
collection of detailed information concerning pesticide
exposure. The statistical power of our study was
enhanced by the large number of cases and controls.
In instances of rare exposures (<1% exposed), we
had limited statistical power to detect
associations. We restricted our analyses of individual
pesticide compounds to those for which at least 1%
of respondents indicated exposure.
The study was not restricted to pesticide exposure
experienced by a specific occupational group.
Occupational exposure was quite diverse; single
versus multiple pesticides; indoor versus
outdoor applications. For example, men who work in
animal confinement buildings, grain elevators, and
pesticide manufacturing have different exposure
patterns in comparison with grain farmers and
commercial applicators. Because this study encompassed a
large geographical area of Canada, there was substantial
diversity among agricultural enterprises and in the
patterns and types of pesticide exposure.
Delineating the putative relationship between exposure to
pesticides and NHL is complicated: (a) by
the subject’s exposure to a variety of different
pesticides many of which are not mutagenic,
teratogenic, or carcinogenic when tested as a single compound;
(b) by the complexity of formulations of
pesticides, the details of which are privileged
proprietary information; (c) by the
diversity of routes of possible exposure, which include
ingestion, dermal, inhalation, and ocular; (d)
by unexpected interactions among seemingly
unrelated exposures, such as the increased permeability
of rubber gloves to 2,4-D when exposed simultaneously to
the insect repellent DEET and sunlight
(46) ; and (e) by the role
of differential genetic susceptibility.
Garry et al. (47) describe a
potential mechanism to explain the relationship
between exposure to specific pesticides and an
increased risk of developing NHL. They have demonstrated
specific chromosomal alterations in the peripheral
lymphocytes of pesticide applicators exposed to a
variety of pesticide classes. A higher frequency of
chromosomal breaks involving band 18q21 was found
in men who applied only herbicides compared with
nonoccupationally exposed controls. Higher
frequencies of rearrangements and breaks involving
band 14q32 were found among men who applied herbicides,
insecticides, and fumigants compared with controls.
Reciprocal translocations between chromosomes 14q32
and 18q21 are frequently found in NHL patients.
Our results support previous findings of an association
between NHL and specific pesticide exposures. Our
strategy of assessing risk by several different
approaches, beginning with general categories (e.g.,
herbicides), proceeding through cumulative
pesticide exposure to specific chemical classes, and
proceeding further to specific chemicals, proved
effective in delineating complex relationships. In
our final models, NHL was associated with a
personal history of cancer; a history of cancer in
first-degree relatives; and exposure to
dicamba-containing herbicides, to mecoprop, and to
aldrin. A personal history of measles and of
allergy desensitization treatments lowered risk.
 |
Acknowledgments |
We are indebted to the members of the Advisory Committee for
this project for the sharing of their experiences (Drs.
G. B. Hill, A. Blair, L. Burmeister, H. Morrison,
R. Gallagher, and D. White); to the provincial
coordinators and data managers for their meticulous
attention to detail (T. Switzer, M. Gantefor, J.
Welyklolowa, J. Ediger, I. Fan, M. Ferron, E. Houle, S. de
Freitas, K. Baerg, L. Lockinger, E. Hagel, P. Wang, and
G. Dequiang), and to Dr. G. Theriault for
supervising the collection of data in Quebec. We
appreciate the care and dedication of S. de Freitas
in preparation of the manuscript. The study participants gave
freely of their time and shared personal details with
us, and we sincerely thank each of them.
 |
Footnotes |
The costs of publication of this
article were defrayed in part by the payment of
page charges. This article must therefore be hereby
marked advertisement in accordance with 18 U.S.C.
Section 1734 solely to indicate this fact.
1 This research
was funded by Health Canada Grant 6608-1258, the
British Columbia Health Research Foundation, and the Centre
for Agricultural Medicine, University of Saskatchewan.

2 To whom
requests for reprints should addressed, at Centre for
Agricultural Medicine, 103 Hospital Drive, P. O. Box
120, Royal University Hospital, Saskatoon, S. K.,
S7N 0W8, Canada. Phone: (306) 966-6154; Fax: (306)
966-8799; E-mail:
mcduffie@sask.usask.ca.

3 Dr. Choi was a
collaborator who is now deceased.

4 The
abbreviations used are: NHL, non-Hodgkin’s lymphoma;
DDT, 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane;
STS, soft tissue sarcoma; HD, Hodgkin’s disease;
MM, multiple myeloma; 2,4-D,
2,4-dichlorophenoxyacetic acid; MCPA,
4-chloro-2-methylphenoxyacetic acid; 2,4,5-T,
2,4,5-trichlorophenoxyacetic acid; OR, odds ratio;
ORadj, adjusted OR; 95% CI, 95% confidence
interval.

5 SPSS-Data Entry
II Statistical Package for the Social Sciences:
Statistical Data Analysis. SPSS Inc., Chicago, Illinois, 1998.

6 EGRET Intuitive
Software for DOS Micros Statistics and Epidemiology
Research Corporation, 1993.

Received 12/20/00; revised 8/13/01; accepted 8/22/01.
 |
References |
- Cantor K. P., Blair A., Everett G., Gibson R.,
Burmeister L. F., Brown L. M., Schuman L., Dick F. R.
Pesticides and other agricultural risk factors for
non-Hodgkin’s lymphoma among men in Iowa, and Minnesota.
Cancer Res., 52: 2447-2455, 1992.[Abstract]
- Saftlas A. F., Blair A., Cantor K. P.,
Hanrahan L., Anderson H. A. Cancer and other causes of death
among Wisconsin farmers. Am. J. Ind. Med., 11:
119-129, 1987.[Medline]
- Pearce N. E., Smith A. H., Fisher D. O.
Malignant lymphoma and multiple myeloma linked with
agricultural occupation in a New Zealand cancer
registration-based study. Am. J. Epidemiol., 121:
225-237, 1985.[Abstract]
- Burmeister L. F., Everett G. D., Van Lier S.
F., Isacson P. Selected cancer mortality and farm practices
in Iowa. Am. J. Epidemiol., 118: 72-77, 1983.[Abstract]
- Cantor K. P. Farming and mortality from
non-Hodgkin’s lymphoma: a case-control study. Int. J.
Cancer, 29: 239-247, 1982.[Medline]
- Delzell E., Grufferman S. Mortality among
white and non-white farmers in North Carolina 1976–78. Am.
J. Epidemiol., 121: 391-402, 1985.[Abstract]
- Buesching D. P., Wallstadt L. Cancer mortality
among farmers. J. Natl. Cancer Inst. (Bethesda), 72:
503-504, 1984.[Medline]
- Schumacher M. C. Farming occupations and
mortality from non-Hodgkin’s lymphoma in Utah: a
case-control study. J. Occup. Med., 27: 580-584,
1985.[Medline]
- Wigle D. T., Semenciw R. M., Wilkins K.,
Riedel D., Ritter L., Morrison H., Mao Y. Mortality study of
Canadian farm operators: non-Hodgkin’s lymphoma mortality
and agricultural practices in Saskatchewan. J. Natl. Cancer
Inst. (Bethesda), 82: 575-580, 1990.[Abstract]
- Hardell L., Eriksson M., Lenner P., Lundgren
E. Malignant lymphoma and exposure to chemicals especially
organic solvents, chlorophenols and phenoxy acids: a
case-control study. Br. J. Cancer, 43: 169-176, 1981.[Medline]
- Hoar S. K., Blair A., Holmes F., Boysen C.,
Robel R. J., Hoover R., Fraumeni J. F. Agricultural
herbicide use and risk of lymphoma and soft tissue sarcoma.
J. Am. Med. Assn., 256: 1141-1147, 1986.[Abstract]
- Woods J. S., Polissar L., Severson R. K.,
Heuser L. S., Kulander E. G. Soft tissue sarcoma and
non-Hodgkin’s lymphoma in relation to phenoxyherbicide and
chlorinated phenol exposure. J. Natl. Cancer Inst., 78:
899-910, 1987.[Medline]
- Zahm S. H., Weisenburger D. D., Babbit P. A.,
Saal R. C., Vaught J. B., Cantor K. P., Blair A. A case
control study of non-Hodgkin’s lymphoma and agricultural
factors in Eastern Nebraska. Epidemiology, 1:
349-356, 1990.[Medline]
- Alavanja M. C. R., Blair A., Merkle S., Teske
J., Eaton B., Reed B. Mortality among forest and soil
conservationists. Arch. Environ. Health, 44: 94-101,
1989.[Medline]
- Gallagher R. P., Threlfall W. J., Band P. R.,
Spinelli J. J. Cancer mortality experience of woodworkers,
loggers, fishermen, farmers and miners in British Columbia.
Natl. Cancer Inst. Monogr., 69: 163-167, 1985.[Medline]
- Kross B. C., Burmeister L. F., Ogilvie L. K.,
Fuortes L. J., Fu C. M. Proportionate mortality study of
golf course superintendents. Am. J. Ind. Med., 29:
501-506, 1996.[Medline]
- Scherr P. A., Hutchison G. B., Neiman R. S.
Non-Hodgkin’s lymphoma and occupational exposure. Cancer
Res., 52 (Suppl.): 5503s-5509s, 1992.[Abstract]
- Devesa S. S., Fears T. Non-Hodgkin’s lymphoma
time trends: United States and international data. Cancer
Res., 52 (Suppl.): 5432s-5440s, 1992.[Abstract]
- Banks P. M. Changes in diagnosis of
non-Hodgkin’s lymphoma over time. Cancer Res., 52
(Suppl.): 5453s-5455s, 1992.[Abstract]
- Holford T. R., Zheng T., Magne S. T., McKay
L. A. Time trends of non-Hodgkin’s lymphoma: are they real?
what do they mean?. Cancer Res., 52 (Suppl.):
5443s-5446s, 1992.[Abstract]
- Dosman J. A., McDuffie H. H., Pahwa P.,
Fincham S., McLaughlin J. R., Robson D., Theriault G. .
Pesticides, Soft Tissue Sarcoma, Lymphoma, and Multiple
Myeloma. A Case Control Study in Three Regions of Canada.
Report to Health and Welfare Canada on Project 6008-1223,
University of Saskatchewan Saskatoon, Canada 1990.
- IARC Working Group. An evaluation of
chemicals and industrial processes associated with cancer in
humans based on human and animal data. Cancer Res., 40:
1-12, 1980.[Abstract]
- IARC. Some halogenated hydrocarbons and
pesticide exposures. In: Monographs on the Evaluation
of the Carcinogenic Risk of Chemicals to Humans, Vol. 41.
Lyon, France: IARC, 1986.
- IARC. Overall Evaluation of Carcinogenicity:
An Updating of IARC Monographs, Volumes 1–42, Suppl. 7.
Lyon, France: IARC, 1987.
- IARC. Occupational exposures in insecticide
application and some pesticides. In: Monographs on
the Evaluation of Carcinogenic Risks to Humans, Vol. 53.
Lyon, France: IARC, 1991.
- Breslow, N. E., and Day, N. E. The analysis
of case-control studies. In: Statistical Methods in
Cancer Research, Vol. 1, IARC Sci. Publ. 32. Lyon, France:
IARC, 1980.
- Bond G. C., Bodner K. M., Cook R. R. Phenoxy
herbicides and cancer: insufficient epidemiologic evidence
for a causal relationship. Fundam. Appl. Toxicol., 12:
172-188, 1989.[Medline]
- Wiklund K., Dich J., Holm L-E. Risk of
malignant lymphoma in Swedish pesticide appliers. Br. J.
Cancer, 56: 505-508, 1987.[Medline]
- Wiklund K., Holm L-E. Trends in cancer risks
among Swedish agricultural workers. J. Natl. Cancer Inst.
(Bethesda), 77: 657-664, 1986.[Medline]
- Cerhan J. R., Wallace R. B., Folsom A. R.,
Potter J. D., Sellers T. A., Zheng W., Lutz C. T. Medical
history risk factors for non-Hodgkin’s lymphoma in older
women. J. Natl. Cancer Inst. (Bethesda), 89: 314-318,
1997.[Abstract]
- Berstein R., Ross R. K. Prior medication use
and health history as risk factors for non-Hodgkin’s
lymphoma: preliminary results from a case-control study in
Los Angeles County. Cancer Res., 52 (Suppl.):
5510s-5515s, 1992.[Abstract]
- Linet M. S., Pottern L. M. Familial
aggregation of hematopoietic malignancies and risk of
non-Hodgkin’s lymphoma. Cancer Res., 52 (Suppl.):
5465s-5473s, 1992.[Abstract]
- Goldgar D. E., Easton D. F., Cannon-Allright
L. A., Skolnick M. H. Systematic population-based assessment
of cancer risk in first degree relatives of cancer probands.
J Natl Cancer Inst. (Bethesda), 86: 1600-1608, 1994.[Abstract]
- Koepsell T. D., Daling J. R., Weiss N. S.,
Taylor S. W., Olshan A. F., Swanson G. M., Child M.
Antigenic stimulation and the occurrence of multiple
myeloma. Am. J. Epidemiol., 126: 1051-1062, 1987.[Abstract]
- Vena J. E., Bona J. R., Byers T. E.,
Middleton E., Swanson M. K., Graham S. Allergy-related
diseases and cancer: an inverse association. Am. J.
Epidemiol., 122: 66-74, 1985.[Abstract]
- Mills P. K., Beeson W. L., Fraser G. E.,
Phillips R. L. Allergy and cancer: organ site-specific
results from the Adventist health study. Am. J. Epidemiol.,
136: 287-295, 1992.[Abstract]
- Severson R. K., Davis S., Thomas D. B.,
Stevens R. G., Heuser L., Sever L. E. Acute myelocytic
leukemia and prior allergies. J Clin. Epidemiol., 42:
995-1001, 1989.[Medline]
- McDuffie H. H., Cockcroft D. W., Talebi Z.,
Klaassen D. J., Dosman J. A. Lower prevalence of positive
atopic skin tests in lung cancer patients. Chest, 93:
241-246, 1988.[Abstract]
- Herrinton L. J., Friedman G. D. Cigarette
smoking and risk of non-Hodgkin’s lymphoma subtypes. Cancer
Epidemiol. Biomark. Prev., 7: 25-28, 1998.[Abstract]
- Brown L. M., Everett G. D., Gibson R.,
Burmeister L. F., Schuman L. M., Blair A. Smoking and risk
of non-Hodgkin’s lymphoma, and multiple myeloma. Cancer
Causes Control, 3: 49-55, 1992.[Medline]
- Linet M. S., McLaughlin J. K., Hsing A. W.,
Wacholder S., CoChien H. T., Schuman L. M., Bjelke E., Blot
W. J. Is cigarette smoking a risk factor for non-Hodgkin’s
lymphoma? results from the Lutheran Brotherhood Cohort
Study. Leuk. Res., 16: 621-624, 1992.[Medline]
- Blair A., Zahm S. H. Epidemiologic studies of
cancer among agricultural populations McDuffie H. H. Dosman
J. A. Semchuk K. M. Olenchock S. Senthilselvan A. eds. .
Agricultural Health and Safety: Workplace, Environment,
Sustainability, : 111-117, CRC Lewis Publishers Boca
Raton, FL 1994.
- Brown L. M., Dosemeci M., Blair A.,
Burmeister L. Comparability of data obtained from farmers
and surrogate respondents on use of agricultural pesticides.
Am. J. Epidemiol., 134: 348-355, 1991.[Abstract]
- Blair A., Zahm S. H. Herbicides and cancer: a
review and discussion of methodologic issues. Recent Results
Cancer Res., 120: 132-145, 1990.[Medline]
- Blair A., Zahm S. H. Methodologic issues in
exposure assessment for case-control studies of cancer and
herbicides. Am. J. Ind. Med., 18: 285-293, 1990.[Medline]
- Moody R. P., Nadeau B. Effect of the mosquito
repellent DEET and long-wave ultraviolet radiation on
permeation of the herbicide 2,4-D and the insecticide DDT in
natural rubber gloves. Am. Ind. Hyg. Assn. J., 53:
436-441, 1992.
- Garry V. F., Tarone R. E., Long L., Griffith
J., Kelly J. T., Burroughs B. Pesticide appliers with mixed
pesticide exposure: G-banded analysis and possible
relationship to non-Hodgkin’s lymphoma. Cancer Epidemiol.
Biomark. Prev., 5: 11-16, 1996.[Abstract]
http://cebp.aacrjournals.org/cgi/content/full/10/11/1155 |