
|

Unintentional Topical Lindane Ingestions --- United
States, 1998--2003
Lindane* is an organochlorine pesticide found in certain
prescription-only shampoos and topical lotions used to treat
pediculosis (i.e., lice infestation) and scabies; lindane has
been associated with human neurologic toxicity (1,2).
In 2004, CDC was alerted to cases of illness caused by
unintentional ingestion of lindane by persons mistaking the
product for a liquid oral medication (e.g., cough syrup). To
assess the extent of illness from ingestion of lindane, CDC,
with assistance from the U.S. Environmental Protection Agency,
Food and Drug Administration (FDA), and state health
departments, collected case reports and analyzed data from the
Sentinel Event Notification System for Occupational
Risks-Pesticides (SENSOR-Pesticides) program and the Toxic
Exposure Surveillance System (TESS). This report summarizes
the results of that analysis, which identified 870 cases of
unintentional lindane ingestion during 1998--2003, and
describes two examples of lindane ingestions. To reduce the
risk of lindane ingestion, public health authorities should
alert clinicians to the hazards of lindane and the importance
of following FDA usage guidelines, which include dispensing
lindane in manufacturer-produced, 1- or 2-ounce single-use
containers.
Case Reports
Case 1. In November 2004, the Washington State
Department of Health reported that a boy aged 3 years ingested
approximately 1 teaspoon of 1% lindane shampoo from a
previously used 2-ounce bottle. Subsequently, the mother
induced vomiting in the boy twice; 1 hour later the boy
collapsed and experienced a tonic-clonic seizure lasting 4--5
minutes. After 3 hours, the child was discharged from the
emergency department in stable condition.
Case 2. In December 2003, a man aged 47 years in
Texas mistakenly ingested 1 ounce of lindane (percentage
concentration unknown) from a bottle he believed to be cough
syrup. The man vomited; he contacted the poison control center
the following morning. He did not seek clinical evaluation.
Surveillance Data
Data were analyzed from pesticide poisoning surveillance
systems participating in the SENSOR-Pesticides program†
to identify symptomatic cases involving unintentional topical
lindane ingestions during 1998--2003. Cases were classified as
definite, probable, possible, or suspicious based on the
clinical interpretation of signs or symptoms reported by a
physician or patient, and evidence of lindane ingestion (3,4).
Cases were also obtained from TESS§, which is
maintained by the American Association of Poison Control
Centers; poison information specialists determined which cases
had signs and symptoms consistent with lindane exposure.
Illness severity was categorized for all cases. Excluded were
cases involving ingestion of veterinary and agricultural
pesticide products that contained lindane.
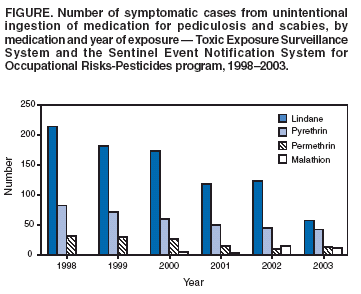
During 1998--2003, TESS reported 857 symptomatic cases of
unintentional lindane ingestion (Figure);
none of the cases were reported as resulting in death.
Severity was low in 778 cases (91%), moderate in 71 cases
(8%), and high in eight cases (1%) (4). Among 823
patients with known ages, median age was 13 years (range:
<1--86 years); 53% were female. Signs and symptoms included
vomiting (59%), nausea (18%), oral irritation (19%), abdominal
cramping (4%), cough (4%), and seizure (3%).
During 1998--2003, SENSOR-Pesticides identified a total of
13 symptomatic cases of unintentional lindane ingestion. Four
cases (31%) were classified as definite, two (15%) as
probable, six (46%) as possible, and one (8%) as suspicious.
Severity was low in eight cases (62%), moderate in three cases
(23%), and high in two cases (15%) (3). Median age was
7 years (range: <1--58 years), and 69% were male. Signs and
symptoms included vomiting (69%), nausea (46%), headache
(23%), seizure (23%), abdominal cramping (8%), and confusion
(8%). Six (46%) cases in children and four (31%) cases in
adults were the result of mistaking lindane for cough syrup;
two (15%) cases were in unsupervised children who drank
lindane, and one (8%) case was the result of pharmacy error
(i.e., lindane was recovered from a bottle labeled albuterol).
In addition to lindane, FDA-approved treatments for
pediculosis include two over-the-counter medications
(pyrethrin/piperonyl butoxide and permethrin) and malathion, a
prescription-only therapy. During 1998--2003, TESS identified
523 symptomatic cases of unintentional ingestion of these
alternative medications (Figure). Median
age was 9 years (range: <1--67 years). Among TESS reports,
unintentional lindane ingestions were more likely to produce
illness (857 illnesses of 1,463 ingestions [58%]) than
unintentional ingestions of each of three other medications,
and more likely to produce illness than all three of those
medications combined (523 illnesses of 1,691 ingestions [31%];
odds ratio = 3.16, 95% confidence interval = 2.72--3.67).
Reported by: J Sievert, Texas Dept of State
Health Svcs. M Lackovic, MPH, Louisiana Dept of Health and
Hospitals. A Becker, PhD, Florida Dept of Health. DH Lew,
Oregon Dept of Human Svcs. B Morrissey, Washington State Dept
of Health. J Blondell, PhD, Office of Pesticide Programs, US
Environmental Protection Agency. LY Kim-Jung, PharmD, MR
Pitts, PharmD, CA Holquist RPh, Food and Drug Admin. AM
Petersen, MPH, JS Alonso-Katzowitz, GM Calvert, MD, Div of
Surveillance, Hazard Evaluations, and Field Studies, National
Institute for Occupational Safety and Health, CDC.
Editorial Note:
Pediculosis and scabies are common human parasitic
infestations. This report indicates that when lindane, a
treatment for pediculosis and scabies, is unintentionally
ingested, illness can occur, including vomiting and seizures.
In 1995, lindane was changed to a second-line therapy for
pediculosis because safer alternatives existed (5).
Lindane also had the slowest pediculicidal and least effective
ovicidal activity compared with three other
approved pediculicides (i.e., 1% permethrin, 0.3% pyrethrin,
and 0.5% malathion) (6). In 2003, in light of continued
postmarketing surveillance reports of toxicity, FDA revised
product labeling guidelines to limit the amount of lindane
dispensed to 1- or 2-ounce single-use containers and to
require providing patients with a Medication Guide warning of
risks from inappropriate use. In addition, FDA issued a Public
Health Advisory with these changes (7). The new
advisory, along with a substantial increase in retail price
for lindane, appear to have resulted in a declining number of
cases of lindane ingestion (Figure). This
decline is similar to the 67% decrease in lindane
prescriptions from 1998 to 2003 (8).
Before the advisory, bottles of bulk lindane were sometimes
repackaged by pharmacies into smaller bottles resembling those
used for liquid oral medications (e.g., cough syrup). This
resemblance likely contributed to many unintentional
ingestions. Subsequent to the advisory, bottles of bulk
lindane still in use were not recalled from pharmacies.
Therefore, some repackaging might still occur. In addition,
consumers might have repackaged lindane in their homes.
In September 2004, the North American Task Force on Lindane
drafted an action plan for future use. On January 1, 2005,
Canada withdrew registration of lindane for agricultural pest
control; Mexico is working on a plan to phase out all uses of
lindane. However, with the exception of California, which
banned lindane for medicinal use on January 1, 2002, U.S.
representatives to the North American Commission for
Environmental Cooperation announced that the United States
will continue to allow use of lindane as both a pesticide and
pharmaceutical (9).
The findings in this report are subject to at least three
limitations. First, because of the passive surveillance
methodology of TESS and SENSOR, the number of reported cases
is likely fewer than the number of actual cases. Second,
certain eligible cases might have been inadvertently excluded
because of erroneous information that suggested exposure to
lindane in a veterinary or agricultural product. Finally,
although all cases were symptomatic, the possibility of false
positives cannot be excluded. Because clinical findings of
lindane poisoning are nonspecific and no standard diagnostic
test exists, certain illnesses related temporally to lindane
exposure might not have been caused by the exposure.
Lindane use in shampoos and lotions for treatment of
pediculosis and scabies is declining. However, because of the
toxicity of lindane and the potential for illness from
unintentional ingestion, health-care providers should be
educated regarding appropriate use and packaging. Lindane is a
second-line therapy for both scabies and lice and should not
be tried unless other treatments have failed or are
intolerable; use of lindane also should be avoided for persons
weighing less than 110 pounds (50 kg). Because of the risk for
toxicity, treatment should not be repeated, even if itching
persists; itching can occur, even after successful treatment
(especially for scabies) and can be treated symptomatically.
In addition, pharmacists should not transfer lindane to other
containers and should only dispense lindane in
manufacturer-provided 1- or 2-ounce containers. Finally,
periodic educational outreach programs can help increase
awareness among health-care providers of the new lindane use
guidelines.
References
- Tenenbein M. Seizures after lindane therapy. J Am
Geriatr Soc 1991;39:394--5.
- Fischer TF. Lindane toxicity in a 24-year-old woman. Ann
Emerg Med 1994;24:972--4.
- Calvert GM, Plate DK, Das R, et al. Acute occupational
pesticide-related illness in the US, 1998--1999:
surveillance findings from the SENSOR-Pesticides program. Am
J Ind Med 2004;45:14--23.
- Calvert GM, Sanderson WT, Barnett M, Blondell JM, Mehler
LN. Surveillance of pesticide-related illness and injury in
humans. In: Krieger R, ed. Handbook of pesticide toxicology.
2nd ed. San Diego, CA: Academic Press; 2001.
- Roberts RJ. Clinical practice: head lice. N Engl J Med
2002;346:1645--50.
- Meinking TL, Entzel P, Villar ME, Vicaria M, Lemard GA,
Porcelain SL. Comparative efficacy of treatments for
pediculosis capititis infestations: update 2000. Arch
Dermatol 2001;137:287--92.
- Center for Drug Evaluation and Research, Food and Drug
Administration. Lindane shampoo and lindane lotion.
Rockville, MD: Food and Drug Administration; 2003. Available
at
http://www.fda.gov/cder/drug/infopage/lindane/default.htm.
- IMS Health. National Prescription Audit Plus™. Plymouth
Meeting, PA: IMS Health; 2005.
- North American Commission for Environmental Cooperation.
Mexico to eliminate toxic chemical lindane. Montreal,
Canada: North American Commission for Environmental
Cooperation; 2004. Available at
http://www.cec.org/news/details/index.cfm?varlan=english&ID=2631.
* Lindane is also referred to as
gamma-hexachlorocyclohexane.
† SENSOR-Pesticides is a surveillance program
coordinated by the National Institute for Occupational Safety
and Health (NIOSH) at CDC and conducted by health departments
in nine states. Most participating states collect information
on both nonoccupational and occupational pesticide poisonings
from various sources (e.g., poison control centers, workers'
compensation agencies, or state departments of agriculture).
However, priority is given to occupational cases; therefore,
the number of nonoccupational poisoning cases is limited.
§ TESS receives reports from nearly all poison
control centers nationwide.
Figure
Return to top.
Use of trade names and commercial sources is
for identification only and does not imply endorsement by
the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided
as a service to MMWR readers and do not constitute
or imply endorsement of these organizations or their
programs by CDC or the U.S. Department of Health and Human
Services. CDC is not responsible for the content of pages
found at these sites. URL addresses listed in MMWR
were current as of the date of publication.
|
All MMWR HTML versions of articles are
electronic conversions from ASCII text into HTML. This
conversion may have resulted in character translation or
format errors in the HTML version. Users should not rely on
this HTML document, but are referred to the electronic PDF
version and/or the original MMWR paper copy for the
official text, figures, and tables. An original paper copy of
this issue can be obtained from the Superintendent of
Documents, U.S. Government Printing Office (GPO), Washington,
DC 20402-9371; telephone: (202) 512-1800. Contact GPO for
current prices.
**Questions or messages regarding errors in formatting
should be addressed to
mmwrq@cdc.gov.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5421a2.htm
Date last
reviewed: 6/2/2005
|